Life gets the green light!
A child with epilepsy, a woman with cancer, a former soldier with depression, and how cannabis transformed their lives.


Unconventional cases require an unconventional perspective.






Breaking news!
Why cannabis in medicine?
1. The mechanism:
We all have a SYSTEM capable of reducing pain, inflammation, anxiety, insomnia, and even seizures.
2. The Happiness Molecule:
Meet Anandamide, also known as the “happiness molecule,” which is the only substance in the world capable of activating the benefits of the system mentioned above.
3. The Discovery:
Cannabis contains the only known substance identical to Anandamide, and therefore, it can also activate the mechanism and its benefits.
But why activate it “just like that”?
The name of this system is ECS (Endocannabinoid System), and it has proven effects on pain, seizures, inflammation, and anxiety.
When activated, as after using cannabis, patients with chronic pain, epilepsy, insomnia, anxiety, and other conditions can experience an almost instantaneous improvement in symptoms!
A decision that brought me hope again
“For years, I slept with one eye open. The fear of another seizure, the crying in the middle of the night, the anguish of not knowing what to do. My son couldn’t find peace… and neither could I.
We tried everything. Doctors, tests, medications that only left him groggy and drained. Then cannabidiol treatment came into our lives.
Today, I watch him playing in the yard, laughing like a child should laugh… and I cry. But this time, tears of relief. Because for the first time in years, my heart is calm.”
The identification has been removed upon request, for reasons of personal discretion.


Less stress, more
well-being
100%
NATURAL
EXTRACTION
WITH CO2
EXPRESS
DELIVERY

Seals and approvals
Logistics of your treatment
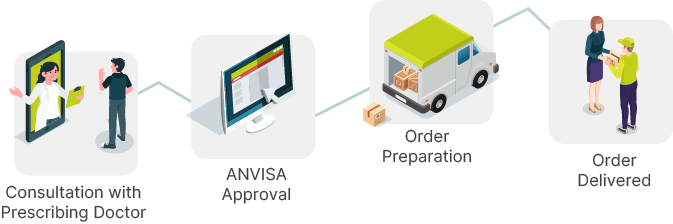

Who confirms the effectiveness of cannabis?

Increasingly common every day!
I am a doctor specialized in child psychiatry and a member of the Knabs medical team. When I opened my practice in 2023, I was surprised by the number of children already using cannabidiol, and those few I was able to follow always highlighted: more quality of life!
Dr. Eduarda Rojo, psychiatrist.
CRM 209829
Who confirms the effectiveness of cannabis?

WHO (World Health Organization) – 2017
Declared CBD safe and non-addictive. Recognized its effectiveness in epilepsy, anxiety, chronic pain, and chemotherapy-induced nausea.

CFM (Federal Medical Council) – 2014
Authorized its use for refractory epilepsy. Studies also show benefits for Parkinson’s, Alzheimer’s, schizophrenia, and insomnia.

Anvisa (National Health Surveillance Agency) – 2019
Regulated the use of CBD with up to 0.2% THC. Approved for Dravet syndrome, Lennox-Gastaut syndrome, and Tuberous Sclerosis, with expanded use under prescription.
+3.5 MILLION
PRODUCTS SOLD
🇺🇸
- Produced on the best farms in Colorado, USA.
- Vegan, gluten-free, and 100% organic.
- Pharmaceutical-grade approved!





Medical Team

Focused on the best applications of medicinal cannabis in the fields of:
- Orthopedics
- Psychiatry
- Geriatrics
- Neurology
- Oncology



OPPORTUNITY
Your first consultation is free!
All stages are our responsibility.
After the consultation and form completion, we take care of all the steps.
FAQ
Frequently Asked Questions
It refers to the use of the Cannabis sativa plant or its derivatives for therapeutic purposes, under medical prescription and supervision.
📚 WHO – Cannabis: Health and Human Rights, 2019
The plant contains various compounds, primarily cannabinoids—especially THC (tetrahydrocannabinol), which is psychoactive, and CBD (cannabidiol), which is non-psychoactive.
📚 WHO – Cannabidiol (CBD) Critical Review Report, 2018
The WHO recognizes scientific evidence for use in:
– Refractory epilepsy
– Chronic pain
– Spasticity related to multiple sclerosis
– Chemotherapy-induced nausea and vomiting
📚 WHO – The Health and Social Effects of Nonmedical Cannabis Use, 2016
Only in specific cases, with clinical support and scientific evidence. It should not be used as a blanket replacement for conventional therapies.
📚 WHO – Expert Committee on Drug Dependence, 2018
Yes. ANVISA authorizes medical use with a prescription. Personal importation is regulated by RDC No. 660/2022, and domestic sales follow RDC No. 327/2019, which sets rules for manufacturing, prescription, and sales in pharmacies.
📚 ANVISA – RDC 660/2022 and RDC 327/2019
They include sublingual oil, capsules, oral sprays, vaporized inhalation, and medications containing purified THC or CBD.
📚 WHO – Cannabis: Health and Human Rights, 2019
CBD does not have addiction potential. THC can lead to dependence, especially with prolonged and unsupervised use.
📚 WHO – The Health and Social Effects of Nonmedical Cannabis Use, 2016
Only physicians legally authorized to practice, in accordance with the health regulations of each country.
📚 WHO – Cannabis: Health and Human Rights, 2019
LINKS
- Frequently Asked Questions
- Privacy Policy
- Terms and Conditions
NOTICE
We inform you that we do not sell cannabidiol oil on this or any other website. This website operates solely to assist with the administrative process between prescription and importation.
According to current legislation, there is no stock available in Brazil. To acquire the product, it is essential that the interested party undergoes a medical consultation and registers with ANVISA as an authorized patient for cannabidiol importation.
Thank you for your understanding.
SECURE SITES


